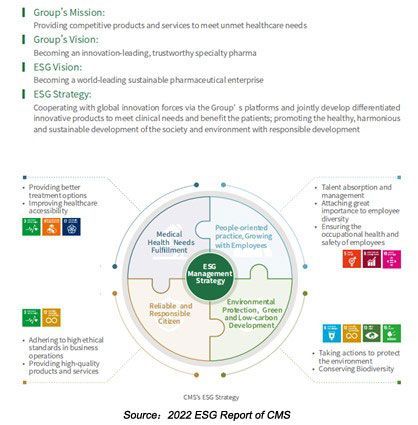
Cambridge, MA, Jul 5, 2023 – (ACN Newswire) – Moderna, Inc. (Nasdaq:MRNA), a biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines, today provided an update on regulatory submissions for mRNA-1345, a vaccine for the prevention of RSV-associated lower respiratory tract disease (RSV-LRTD) and acute respiratory disease (ARD) in adults aged 60 years or older.
The Company has submitted marketing authorization applications for mRNA-1345 with the European Medicines Agency (EMA), Swissmedic in Switzerland, and the Therapeutic Goods Administration (TGA) in Australia and has initiated the rolling submission process for a Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA) for the licensure of the mRNA-based RSV vaccine.
"We are proud to announce these filings for the use of our RSV vaccine candidate, mRNA-1345, in the European Union, Switzerland, Australia, and the U.S. RSV is a major cause of lower respiratory tract infections in older adults and can cause a significant burden to health systems through hospitalizations and emergency care admissions," said Stephane Bancel, Chief Executive Officer of Moderna. "Our mRNA platform has allowed us to move from initial clinical testing to our first international Phase 3 trial to initiation of regulatory submissions for mRNA-1345 in just two years, enabling us to tackle this pervasive public health burden with speed and clinical rigor. mRNA-1345 represents the second product coming from our mRNA platform to seek global approval, and with recent positive data in rare disease and cancer, we expect more in the future – further demonstrating the tremendous potential of mRNA to combat disease."
The regulatory applications are based on positive data from a prespecified interim analysis of the pivotal ConquerRSV study, a randomized, double-blind, placebo-controlled study of approximately 37,000 adults 60 years or older in 22 countries. The primary efficacy endpoints were based on two definitions of RSV-LRTD, defined as either two or more symptoms or three or more symptoms of disease. The trial met both its primary efficacy endpoints, with a vaccine efficacy (VE) of 83.7% (95.88% CI: 66.1%, 92.2%; p<0.0001) against RSV-LRTD as defined by two or more symptoms, and a VE of 82.4% (96.36% CI: 34.8%, 95.3%; p=0.0078) against RSV-LRTD defined by three or more symptoms. The vaccine was well tolerated with a favorable safety profile. Most solicited adverse reactions were mild or moderate, and the most commonly reported solicited adverse reactions in the mRNA-1345 group were injection site pain, fatigue, headache, myalgia, and arthralgia. The ConquerRSV study is ongoing, and additional efficacy analyses are planned as cases accrue, including for severe RSV. In addition to older adults, mRNA-1345 is being investigated in a fully enrolled, ongoing Phase 1 trial in pediatric populations.
In January 2023, the U.S. FDA granted mRNA-1345 Breakthrough Therapy Designation for the prevention of RSV-LRTD in adults aged 60 years or older, and mRNA-1345 was previously granted Fast Track designation by the U.S. FDA in August 2021. In Australia, the TGA submission will be evaluated under the Priority Pathway, following approval of the Priority Determination application for mRNA-1345 in April 2023.
Moderna's respiratory disease vaccine pipeline includes Phase 3 trials against influenza and a next-generation COVID-19 candidate. The pipeline also includes four additional influenza vaccines with expanded antigens, vaccines against other respiratory pathogens, and five combination vaccine programs.
About mRNA-1345
mRNA-1345 is an investigational RSV vaccine that consists of a single mRNA sequence encoding for a stabilized prefusion F glycoprotein. The vaccine uses the same lipid nanoparticles (LNPs) as in the Moderna COVID-19 vaccines. The F glycoprotein is on the surface of the virus and is required for infection by helping the virus to enter host cells. It exists in two states, prefusion and postfusion. The prefusion conformation is a significant target of potent neutralizing antibodies, and the protein sequences are largely similar across both RSV-A and RSV-B subtypes.
About Moderna
In over 10 years since its inception, Moderna has transformed from a research-stage company advancing programs in the field of messenger RNA (mRNA), to an enterprise with a diverse clinical portfolio of vaccines and therapeutics across seven modalities, a broad intellectual property portfolio and integrated manufacturing facilities that allow for rapid clinical and commercial production at scale. Moderna maintains alliances with a broad range of domestic and overseas government and commercial collaborators, which has allowed for the pursuit of both groundbreaking science and rapid scaling of manufacturing. Most recently, Moderna's capabilities have come together to allow the authorized use and approval of one of the earliest and most effective vaccines against the COVID-19 pandemic.
Moderna's mRNA platform builds on continuous advances in basic and applied mRNA science, delivery technology and manufacturing, and has allowed the development of therapeutics and vaccines for infectious diseases, immuno-oncology, rare diseases, cardiovascular diseases, and auto-immune diseases. Moderna has been named a top biopharmaceutical employer by Science for the past eight years. To learn more, visit www.modernatx.com.
Moderna Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including regarding: the regulatory process with respect to mRNA-1345, including the potential for regulatory approval; Moderna's plans for further regulatory submissions for mRNA-1345 worldwide; the efficacy and safety and tolerability profile of mRNA-1345; the ongoing ConquerRSV study; and Moderna's expectations regarding future potential products, including in the areas of rare disease and cancer. The forward-looking statements in this press release are neither promises nor guarantees, and you should not place undue reliance on these forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, many of which are beyond Moderna's control and which could cause actual results to differ materially from those expressed or implied by these forward-looking statements. These risks, uncertainties, and other factors include those other risks and uncertainties described under the heading "Risk Factors" in Moderna's Annual Report on Form 10-K for the fiscal year ended December 31, 2022, filed with the U.S. Securities and Exchange Commission (SEC) and in subsequent filings made by Moderna with the SEC, which are available on the SEC's website at www.sec.gov. Except as required by law, Moderna disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this press release in the event of new information, future developments, or otherwise. These forward-looking statements are based on Moderna's current expectations and speak only as of the date of this press release.
Moderna Contacts
Media:
Luke Mircea-Willats
Senior Director, International Communications
Luke.mirceawillats@modernatx.com
Investors:
Lavina Talukdar
Senior Vice President & Head of Investor Relations
617-209-5834
Lavina.Talukdar@modernatx.com
Copyright 2023 ACN Newswire. All rights reserved. http://www.acnnewswire.com