HONG KONG, Mar 8, 2023 – (ACN Newswire) – Essex Bio-Technology Ltd ("Essex" or the "Group", Stock Code: 1061.HK) today announced the annual results for the year ended 31 December 2022.
Financial Performance
During the year under review, the financial results of the Group, have been negatively impacted and ongoing clinical trial programmes have been delayed due to disruptions resulted from sporadic emergence and persistent spread of COVID-19 and the ensuing lockdowns instituted under the "zero-COVID policy" in the PRC. As of the date of the results announcement, the clinical operations of hospitals and outpatient clinics progressively resumed to normalcy.
For the year ended 31 December 2022, the Group achieved a consolidated revenue of approximately HK$1,318 million, with a net profit of approximately HK$225 million. The net profit was weighed down by an impairment loss of approximately HK$ 25.8 million on goodwill arising from the acquisition of YesDok Pte Ltd and its wholly-owned subsidiary in Indonesia.
As of 31 December 2022, the Group had cash and cash equivalents of approximately HK$544 million (2021: approximately HK$671 million). The Board is pleased to propose a final dividend of HK$0.025 (2021: HK$0.055) per ordinary share to be approved at the upcoming annual general meeting of the Group. Together with the interim dividend of HK$ 0.04 per ordinary share paid on 21 September 2022, the total dividend for 2022 would be HK$ 0.065 (2021: HK$ 0.095) per ordinary share.
Revenue of Ophthalmology and Surgical Segments
The Group's revenue is primarily made up of the segments of Ophthalmology and Surgical (wound healing). The revenue of Ophthalmology is approximately HK$554 million, accounted for approximately 42.0% of the Group's revenue, while the revenue of Surgical is approximately HK$764 million, representing approximately 58.0% of the Group's revenue. The core products that are as current growth driver under each segment are:
1. Ophthalmology – Beifushu series (Beifushu eye drops, Beifushu eye gel and Beifushu unit-dose eye drops), Tobramycin Eye Drops, Levofloxacin Eye Drops, Sodium Hyaluronate Eye Drops and Shilishun (Iodized Lecithin Capsules); and
2. Surgical (Wound care and healing) – Beifuji series (Beifuji spray, Beifuji lyophilised powder and Beifuxin gel), Carisolv dental caries removal gel, Dr. YaDian mouth wash and Yi Xue An Granules.
Significant Business Development Activities
The Group is committed to pragmatically investing in new products and technologies to strengthen the Group's product and research and development ("R&D") pipeline as near to mid-term growth driver in ophthalmology and long-term plan for new therapeutics in oncology. During the period under review, significant milestones achieved under business development activities are outlined as follows:
Through acquisition, Shilishun became the company's new added core product
On 8 March 2022, the acquisition of intellectual property rights relating to technologies and process of product R&D, production and right of Marketing Authorisation Holder of Shilishun (Iodized Lecithin Capsules) was completed and Shilishun (Iodized Lecithin Capsules) is being regarded as one of the Group's core products since then.
Secured Exclusive Global Rights and Interests of SkQ1 in the field Ophthalmology from Mitotech
In order to provide the Group with flexibility and independence in the continuing development of the US FDA VISTA programme in the field of dry eye disease and allow the Group to explore further the development of products for other ophthalmic indications to meet the clinical and commercial needs of the Global (as defined below) market, on 13 October 2022, the Group successfully secured (i) a patent assignment deed (the "Patent Assignment Deed"); and (ii) a patent and know-how licence agreement (the "Patent and Know-how Licence Agreement", together with the Patent Assignment Deed, the "Agreements") relating to SkQ1 in the field of ophthalmology from Mitotech.
Pursuant to the Patent Assignment Deed, Mitotech agreed to assign to the Group all the rights of a list of inventions and patents relating to SkQ1 in the field of ophthalmology and all ophthalmic indications.
Pursuant to the Patent and Know-how Licence Agreement, Mitotech agreed to grant the Group an exclusive, transferable and irrevocable Global licence to use a list of patents owned by Mitotech relating to SkQ1 to develop, manufacture, sell and supply any therapeutic products or therapies applied to the eye and its adnexa (the "Products"), including the full global (excluding Armenia, Belarus, Kazakhstan, Kyrgyzstan and Russia) ("Global") right to apply for and obtain patents, to apply for and obtain Global regulatory approval for clinical trials, and to obtain marketing authorisation in relation to the Products.
Following the acquisition of the intellectual property rights relating to SkQ1 on 13 October 2022, the Group's priority is to complete the transfer of chemistry, manufacturing and controls (CMC), know-how and intellectual property rights relating to SkQ1. Concurrently, the Group is re-establishing the VISTA programme with regulators for mitigating any identifiable risks before continuing with the clinical trial. According to Frost & Sullivan, the number of patients with moderate-to-severe dry eye disease alone was around 120 million in the PRC in 2020.
The potential market size of the SkQ1 Product is enormous.
EB12-20145P (HLX04-O) global phase 3 clinical study makes significant progress
In 2020, the Group entered into a co-development and exclusive license agreement with Shanghai Henlius Biotech, Inc. to co-develop a pharmaceutical product EB12-20145P, a recombinant anti-vascular endothelial growth factor ("anti-VEGF") humanized monoclonal antibody injection for the treatment of exudative (wet) age-related macular degeneration ("wet-AMD"). During the period under review, the product has been approved to commence the phase 3 clinical trial in Australia, the United States, Singapore, Russia, Serbia and European Union countries such as Hungary, Spain, Latvia, the Czech Republic and Poland. Also, the first patient has been dosed in the phase 3 clinical study of EB12-20145P for the treatment of wet-AMD in the PRC, Latvia, Australia and the United States.
In February 2023, the Group entered into an amendment agreement with Henlius to amend certain terms of the Co-Development License Agreement, which include payments for regulatory and commercial sales milestones and development costs in respect of the Anti-VEGF Licensed Product, details of which are in the announcement dated 22 February 2023 and the annual results announcement on 8 March 2023.
The Anti-VEGF Licensed Product can be used for treating wet-AMD, diabetic macular edema, macular edema caused by retinal vein occlusion and myopic choroidal neovascularisation. According to Frost & Sullivan, the estimated number of patients of these 4 categories of disease is over 15.8 million in the PRC in 2020. Assuming each patient applies 4 doses in the first year of treatment and 2 to 3 doses in subsequent years, the potential market size of the product is enormous.
Honors and Awards Obtained In 2022
The Group has been included in 2022 Forbes Asia's Best Under A Billion list, a testimony to the Group's achievements to date. Forbes Asia's Best Under A Billion list spotlights 200 top-performing publicly listed small and mid-sized companies in the Asia-Pacific region with annual sales under US$1 billion. In addition, the Group was conferred with China Excellent IR – The Best Shareholder Relationship Award and The Best ESG Award. In addition, Zhuhai Essex Bio-Pharmaceutical Company Limited, a wholly-owned subsidiary of the Group, has been recognised as one of the 2021 top 10 pharmaceutical and health manufacturing companies in Zhuhai, and has also been recognised as one of the 2021 top 100 chemical pharmaceutical companies in the PRC. The Group's Beifushu has been awarded as one of the Chinese reputable medicine brands in four consecutive years. This is a testament to the recognition by the industry for the efficacy and quality of our flagship biologic drug.
Market Development Entrenched Market Access Capability
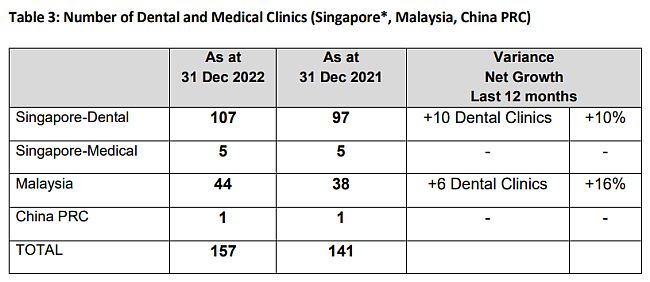
The Group has been relentlessly investing in establishing and strengthening its market access capability. As of 31 December 2022, the Group maintains a network of 43 regional sales offices in the PRC and a total number of about 1,240 sales and marketing representatives, covering more than 10,900 hospitals and medical providers, coupled with approximately 2,130 pharmaceutical stores, which are widely located in the major cities, provinces and county cities in the PRC. Sales to lower-tier cities is supplemented by on-line platform for medical consultation and e-prescription, the on-line platform is further deployed for serving patients with chronic diseases.
The Group's expansion of its market access into Southeast Asian countries via its base in Singapore has been gaining good development traction since 2020.
Research and Development
During the period under review, the Group remains focused executing its 5-year (2021 to 2025) R&D's development plans. As at the date of the announcement, there are 16 R&D programmes in the pre-clinical to clinical stage, out of which the following 4 ophthalmology programmes (inclusive of a new addition of EB11-21148P in 2022) are in late clinical stage and as mid-term growth drivers:
— EB11-18136P: SkQ1 eye drops, second phase 3 clinical trial (US FDA) (VISTA-2) topline data released on 24 February 2021
— EB11-15120P: Azithromycin eye drops, ongoing review by external experts (National Medical Products Administration ("NMPA") in the PRC)
— EB12-20145P: Bevacizumab for wet age-related macular degeneration ("wet-AMD"), phase 3 clinical trial (US FDA, European Medicines Agency, Therapeutic Goods Administration and NMPA in the PRC)
— EB11-21148P: Cyclosporine eye drops, phase 2 clinical trial (NMPA in the PRC)
The Group holds a total of 69 patent certificates or authorisation letters, which include 50 invention patents, 14 utility model patents and 5 design patents. The Group currently has diversified its R&D resources to multiple research sites in Zhuhai (PRC), Boston (United States), London (United Kingdom) and Singapore which support not only our pursuit of new therapeutics but also our recruitment of global talents.
Mr. Patrick Ngiam, Chairman of Essex, said, "Despite yet another difficult year inflicted by the pandemic of COVID-19 on us all, the tenacity, drive and leadership in our DNA were able to deliver sustained stakeholder value. Barring any unforeseen circumstances, being resilient, relevant and growth ready, the Group is optimistic of delivering progressive results.
I would like to take this opportunity to express my sincere gratitude to all stakeholders, business associates and valued customers for the trust, support and cooperation accorded to us, and each and every member of the Group for their relentless efforts rendered in shaping the Group into being a progressive and promising pharmaceutical player."
Full version of Essex's FY2022 Annual Results Announcement can be downloaded at:
https://www1.hkexnews.hk/listedco/listconews/sehk/2023/0308/2023030800766.pdf
About Essex (1061.HK)
Essex Bio-Technology Limited is a bio-pharmaceutical company that develops, manufactures and commercialises genetically engineered therapeutic b-bFGF (FGF-2), having six commercialised biologics marketed in China since 1998. Additionally, it has a portfolio of commercialised products of preservative-free unit-dose eye drops and Shilishun(Iodized Lecithin Capsules) etc.. The products of the Company are principally prescribed for the treatment of wounds healing and diseases in Ophthalmology and Dermatology, which are marketed and sold through approximately 10,900 hospitals and managed directly by its 43 regional sales offices in China. Leveraging on its in-house R&D platform in growth factor and antibody, the Company maintains a pipeline of projects in various clinical stages, covering a wide range of fields and indications.
Media Enquiry:
Strategic Financial Relations Limited (Website: http://www.sprg.com.hk)
Shelly Cheng +852 2864 4857 shelly.cheng@sprg.com.hk
Yan Li +852 2114 4320 yan.li@sprg.com.hk
June Tuo +852 2864 4848 june.tuo@sprg.com.hk
Angela Shen +852 2864 4870 angela.shen@sprg.com.hk
Media: media@essex.com.cn
Investor Enquiry:
Investor Relations: investors@essex.com.cn
Copyright 2023 ACN Newswire. All rights reserved. http://www.acnnewswire.com