HONG KONG, Jul 13, 2022 – (ACN Newswire) – It is reported that Citi initiated coverage on Prenetics Global (PRE.US) with "Buy" rating. Target price is at USD8.10, apply 3x P/S 2023E, at parity with the industry average.
As Covid-19 variants have continued to spread, Citi believes Covid-19 testing will remain a solid cash cow business with good margins and stable cash flows that can help fuel Prenetics' expansion into new businesses, becoming a near-term driver. Citi expects long-term growth will be driven by personalized healthcare test products and from future M&A.
Prenetics is well-positioned to capture the growing consumer interest in personalized health testing. Citi expects Prenetics would deliver a 3-year 26% CAGR growth to 2024E in its Prevention segment, by launching new products from pipeline (e.g. Circle Medical), entering into cancer screening and IVD.
Headquartered in Hong Kong, Prenetics is a major global diagnostics and genetic testing company, with operations across 9 locations, including the United Kingdom, South Africa, India and Southeast Asia. Prenetics offers a wide portfolio of personalized healthcare tests including DTC-GT, cancer screening, and Covid-19 testing.
Copyright 2022 ACN Newswire. All rights reserved. http://www.acnnewswire.com
Tag: Healthcare & Pharm
FDA Grants De Novo Marketing Authorization to Apollo Endosurgery for Apollo ESG and Apollo REVISE, New Endoscopic Systems for Patients with Obesity

AUSTIN, TX, Jul 13, 2022 – (ACN Newswire) – Apollo Endosurgery, Inc. ("Apollo") (NASDAQ:APEN), a global leader in minimally invasive medical devices for gastrointestinal and bariatric procedures, announced today the marketing authorization of the Apollo ESG(TM), Apollo ESG Sx(TM), Apollo REVISE(TM) and Apollo REVISE Sx(TM) Systems through the U.S. Food and Drug Administration's (FDA) De Novo Classification process, a rigorous pre-market review pathway for low-to moderate-risk devices without a predicate. These are the first and only devices authorized by the FDA for endoscopic sleeve gastroplasty (ESG) and endoscopic bariatric revision.
ESG is an incisionless procedure that utilizes an endoscopic suturing system to reduce the volume of a person's stomach and delay emptying of the stomach, resulting in clinically meaningful, durable weight loss. In a randomized controlled trial, the ESG procedure demonstrated safety and effectiveness with durability out to two years.[1] The results from this trial add to a larger body of evidence reporting outcomes in over 10,000 patients receiving ESG. ESG can be performed as a same-day procedure without incisions or scars, and patients typically return to work within a few days.
Bariatric revision procedures are the fastest growing segment of the bariatric surgery market.[2] Studies have shown that after ten years, patients who underwent gastric bypass have regained an average of 20-30% of the weight they initially lost.[3] Transoral outlet reduction (TORe) is an endoscopic procedure performed to revise a previous gastric bypass and like ESG, can be performed as a same-day procedure without incisions or scars.
"The Apollo ESG and Apollo REVISE systems offer a compelling mix of effectiveness, safety, durability, and convenience for treatment of patients with obesity," said Chas McKhann, President and CEO of Apollo. "The authorization of these new endoscopic systems represents a major step forward in addressing the global obesity epidemic."
Obesity, defined as BMI >30 kg/m2, is a complex, chronic disease that affects 650 million adults globally.[4] In the US, 108 million adults and 42% of the population suffer from obesity[5], which is expected to increase in prevalence by 33% in the coming decades.[6] Obesity contributes to an estimated $147-210 billion in annual healthcare expenditures and is associated with many comorbid conditions.[7] Obesity is considered a major risk factor for type 2 diabetes; the CDC cites that 62% of people with diabetes are obese.[5] Obesity is also a risk factor for heart disease, high blood pressure, liver disease, osteoarthritis, and many types of cancer.[7] The primary treatment for obesity is weight loss, though currently, less than 0.2% of adults with obesity are treated surgically for obesity, leaving a substantial unmet need.[2],[8]
Apollo ESG and Apollo REVISE join Apollo's growing portfolio of endoscopic solutions for weight loss in patients with obesity. The Apollo ESG and Apollo ESG Sx Systems are intended to be used by trained gastroenterologists or surgeons to facilitate weight loss in adults with obesity with Body Mass Index (BMI) between 30-50 kg/m2 who have not been able to lose weight or maintain weight loss through more conservative measures. The Apollo REVISE and Apollo REVISE Sx Systems are intended to be used by trained gastroenterologists or surgeons that perform bariatric procedures to facilitate weight loss in adult patients with obesity with BMI between 30-50 kg/m2 by enabling transoral outlet reduction (TORe) as a revision to a previous bariatric procedure. The systems are used with a dual channel endoscope (Apollo ESG and Apollo REVISE) or single channel endoscope (Apollo ESG Sx and Apollo REVISE Sx).
For more information regarding the De Novo marketing authorization of these systems, visit www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/denovo.cfm, which may be updated to reflect this decision at a future date.
About Apollo Endosurgery, Inc.
Apollo Endosurgery, Inc. is a medical technology company focused on development of next-generation, minimally invasive devices to advance therapeutic endoscopy designed to treat a variety of gastrointestinal conditions, including closure of gastrointestinal defects, managing gastrointestinal complications, and weight loss as a treatment of obesity. Apollo's device-based therapies are an alternative to invasive surgical procedures, thus lowering complication rates and reducing total healthcare costs. Apollo's products are offered in over 75 countries today and include the OverStitch(R) Endoscopic Suturing System, the OverStitch Sx(TM) Endoscopic Suturing System, the Orbera(R) Intragastric Balloon System, the X-Tack(R) Endoscopic HeliX Tacking System, as well as the Apollo ESG(TM), Apollo ESG Sx(TM), Apollo REVISE(TM) and Apollo REVISE Sx(TM) Systems.
Apollo's common stock is traded on NASDAQ Global Market under the symbol "APEN". For more information regarding Apollo Endosurgery, go to: www.apolloendo.com.
About Apollo ESG(TM) and Apollo REVISE(TM)
The Apollo ESG(TM) and Apollo REVISE(TM) Systems are intended to facilitate weight loss in patients with obesity (BMI 30-50 kg/m2) and are the first and only devices to be authorized by the FDA for performance of ESG and TORe, respectively. ESG and TORe are performed endoscopically without incisions or scars, allowing patients typically to go home the same day. More than 25,000 ESGs and more than 10,000 endoscopic revision procedures have been performed worldwide by gastroenterologists and surgeons. To review the full indications for use, visit www.apolloendo.com/dfus.
Legal Notice Regarding Forward-Looking Statements
Certain statements in this press release are forward-looking statements that are subject to risks and uncertainties that could cause results to be materially different than expectations. In addition, there is uncertainty about the spread of the COVID-19 virus and the impact it may have on the Company's operations, the Company's financial outlook for future periods, the demand for the Company's products, the Company's liquidity position, global supply chains and economic activity in general. Important factors that could cause actual results to differ materially include: adverse events related to the Company's products, outcomes of clinical studies related to the Company's products, development of competitive medical products by competitors, regulatory approvals and extensive regulatory oversight by the FDA or other regulatory authorities, unfavorable media coverage related to the Company's products or related procedures, coverage and reimbursement decisions by private or government payors, the Company's ability to support the adoption of its products and broaden its product portfolio as well as other factors detailed in Apollo's periodic reports filed with the Securities and Exchange Commission, or SEC, including its Form 10-K for the year ended December 31, 2021 and its Form 10-Q for the period ended March 31, 2022. Copies of reports filed with the SEC are posted on Apollo's website and are available from Apollo without charge. These forward-looking statements are not guarantees of future performance and speak only as of the date hereof, and, except as required by law, Apollo disclaims any obligation to update these forward-looking statements to reflect future events or circumstances.
[1] Abu Dayyeh B, et al. Endoscopic sleeve gastroplasty impact on obesity and comorbidities: results from a US prospective, multicenter, randomized clinical trial with 104 weeks follow-up. Digestive Disease Week; May 24, 2022. Oral presentation.
[2] ASMBS. Estimates of bariatric surgery numbers, 2011-2020.
[3] Adams TD, et al. Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med 2017;377:1143-55.
[4] World Health Organization, June 2021
[5] Centers for Disease Control and Prevention
[6] Finkelstein E, et al. Obesity and Severe Obesity Forecasts Through 2030
[7] Obesity Action Coalition
[8] O'Brien P. Surgical Treatment of Obesity. Endotext (Internet)
CONTACT:
Apollo Endosurgery, Inc.
Jeff Black, Chief Financial Officer, 512-279-5126
investor-relations@apolloendo.com
Darrow Associates Investor Relations
Matt Kreps, 214-597-8200
mkreps@darrowir.com
Copyright 2022 ACN Newswire. All rights reserved. http://www.acnnewswire.com
Malaysian Genomics Forms Partnership for Expansion in Saudi Arabia

PETALING JAYA, Malaysia, Jul 13, 2022 – (ACN Newswire) – Malaysian Genomics Resource Centre Berhad (Bursa: MGRC, 0155), a leading genomics and biopharmaceutical specialist, announced today that it has entered into a strategic partnership with United Doctors Hospital (UDH) for collaboration in genomics and cell therapies.
 |
UDH, located in Jeddah, Saudi Arabia, is accredited by the Saudi Central Board for Accreditation of Healthcare Institutions. UDH has also won numerous awards for social responsibility, including the King Khalid Award for Social Responsibility in 2018, and is certified with the SA8000, the world's leading social certification programme, by Social Accountability International (formerly known as Social Accountability Accreditation Services), a United States-based charitable organisation, in 2016.
Under the partnership, which is valid for three years, Malaysian Genomics and UDH will take the necessary steps to encourage and promote cooperation in the sales and marketing of genetic screening tests and cell therapy products; collaboration in research and development (R&D), and other areas of cooperation in the genomics and cell therapies to be mutually decided by both parties.
Encik Azri Azerai, Executive Director of Malaysian Genomics, said, "This strategic partnership with UDH enables us to expand the geographical reach of our products and services beyond Malaysia to the Middle East and North Africa region. It will also enable us to collaborate on R&D with various domain specialists to enhance our suite of genetic screening tests and cell therapies."
Dr. Hefny Moustafa Hefny, Medical Doctor at UDH, said, "Among the areas being explored by Malaysian Genomics and UDH are opportunities to develop genetic screening tests for hereditary conditions and diseases that are prevalent in the Middle East or Northern Africa, which may be different from other parts of the world."
Malaysian Genomics is actively expanding its business-to-consumer channels as part of the Group's growth strategy. The Group recently acquired a 51% stake in kidney dialysis operator Aquahealth Sdn Bhd to offer personalised kidney care that also involves nutrition management, lifestyle changes, and genetic testing. It is also collaborating with AirAsia's Asean Super App online shopping platform and Speedoc to provide genetic screening services.
Malaysian Genomics Resource Centre Berhad: 0155 [BURSA: MGRC] [RIC: MGRC:KL] [BBG: MGRC:MK], http://www.mgrc.com.my/
Copyright 2022 ACN Newswire. All rights reserved. http://www.acnnewswire.com
Novotech Client SK bioscience Achieves SKYCovione(TM) COVID-19 Vaccine Approval in Korea

SEOUL, S.KOREA, Jul 13, 2022 – (ACN Newswire) – Novotech, the leading Asia Pacific biotech specialist CRO which has recently expanded its services to the US, congratulates its client SK bioscience on the recent COVID-19 vaccine licence approval.
SK bioscience and GSK recently announced the successful authorization:
SK bioscience announced that 'SKYCovione(TM),' South Korea's first COVID-19 vaccine candidate adjuvanted with GSK's pandemic adjuvant has been authorized by the Korean Ministry of Food and Drug Safety (KMFDS). South Korea has become one of the few countries in the world to have both a domestically developed COVID-19 vaccine and a treatment.
SKYCovione(TM) is a self-assembled nanoparticle vaccine targeting the receptor binding domain of the SARS-CoV-2 Spike protein for the parental SARS-Cov-2, jointly developed with the Institute for Protein Design (IPD) at the University of Washington School of Medicine with combination of GSK's pandemic adjuvant. The development of SKYCovione(TM) has been supported by funding from the Bill & Melinda Gates Foundation and Coalition for Epidemic Preparedness Innovations (CEPI).
The results of the Phase III clinical trial, collected in 4,037 adults over 18-year-old, showed that SKYCovione(TM) induced neutralizing antibody responses, against the SARS-CoV-2 parental strain. The neutralizing antibody titres increased about 33 times compared to before the injection and were 3 times that of AstraZeneca's Vaxzevria(TM), the control vaccine used in the study, 2 weeks after the second dose.
The clinical trial was conducted in cooperation with 16 institutions, including Korea University Guro Hospital and IVI (International Vaccine Institute), a non-profit international organization.
SK bioscience will apply for authorizations at other selected regulatory agencies for distribution of SKYCovione(TM), including through the COVAX Facility and for emergency use listing (EUL) to the World Health Organization (WHO).
SKYCovione(TM) is based on recombinant protein vaccine technology which has been used for development of current vaccines including influenza, hepatitis B, and HPV. SKYCovione(TM) can be stored in normal refrigeration conditions from 2 to 8 degrees Celsius, so it is particularly suitable for use in low-income settings without the need for ultra-cold chain facilities.
The market expects that SKYCovione(TM) will accelerate securing of Korea's vaccine sovereignty and reducing dependence on vaccine imports.
Jaeyong Ahn, CEO of SK bioscience said, "The development of Korea's first COVID-19 vaccine was achieved based on the efforts of the government and members who have been working hard all day and night. We will continue to work with various global organizations based on our own research and manufacturing technologies to preemptively respond to new pandemics."
See full press release here https://www.skbioscience.co.kr/en/news/news_01_01?mode=view&id=132&
Dr. John Moller, CEO Novotech said, "As the lead CRO managing the Phase lll study in six APAC countries, the entire team at Novotech congratulates SK bioscience for such an outstanding clinical success and subsequent licence approval. Licence approval is the ultimate goal in clinical research and drug development and we are honoured to be part of the process of bringing new life-saving therapies to market. We look forward to working with SK bioscience on further vaccine developments in the near future."
About Novotech
Novotech is the leading Asia-Pacific biotech specialist CRO. Novotech has integrated labs and phase I facilities and provides drug development consulting and clinical development services across all phases. It has been instrumental in the success of approximately 4,000 clinical trials across a broad range of therapeutic areas. Novotech is well-positioned to serve biopharma clients conducting clinical trials in Asia-Pacific and the US. For more information visit https://novotech-cro.com/contact
Media Contact
David James
E: communications@novotech-cro.com
AU: +61 2 8218 2144
USA: +1 415 951 3228
Asia: +65 3159 3427
Copyright 2022 ACN Newswire. All rights reserved. http://www.acnnewswire.com
Perfect Medical’s Major Shareholders Further Increase along with Company Buybacks, Strong Confidence in the Future Prospects
HONG KONG, Jul 8, 2022 – (ACN Newswire) – Perfect Medical Health Management Limited ("Perfect Medical" or the "Company", the "Group"; Stock code: 1830.HK), one of the largest medical groups in Hong Kong, together with its subsidiaries (collectively referred to as the "Group"), is pleased to announce that on the 4th and 5th July 2022, Dr. Au-Yeung Kong, the executive director, chairman and chief executive officer of the Group ("Dr. Au-Yeung") further purchase a total of 7,5000,000 shares of the Company, for an aggregate consideration of HK$30.675 million. Following the further purchase, Dr. Au-Yeung is interested in an aggregate of 909,287,383 shares and his equity stake increased from 72.92% to 73.33%. At the same time, on the 4th of July 2022, the Company repurchased 1,000,000 shares for an aggregate consideration of HK$3.978 million under the Repurchase Mandate, representing approximately 0.08% of the issued share capital.
The Company announced its FY2021/22 annual results in late June. The Company achieved a historical high revenue of HK$1.35 billion, representing a growth of 23.9% despite the impact of the pandemic. The Company achieved a net profit of HK$305.2 million. If excluding the government subsidies in both years, the revised net profit for the Group increased by 29.8%. With a solid financial position, actively seeking mergers and acquisitions of medical projects and global expansion strategies, the Company will deliver sustainable growth in the long run.
Dr. Au-Yeung, said that "the further purchase demonstrates the confidence about the future prospects of the Company and is optimistic about the Company's future development and may consider further increasing the shareholding in the Company when appropriate in the future. With the weakening of the impact of the pandemic, the Company is well-positioned to capitalize on the market opportunities and respond to the rebound of customers' demand in the post-pandemic era. In the future, the Company will gear up its effort organically, actively seek mergers and acquisitions of medical projects, and make optimization and integration to offer additional high-quality services to our customers. Looking ahead, the Company will increase the proportion of medical services and proceed with the international business expansion, with a view to becoming a truly multinational medical group."
About Perfect Medical Health Management Limited
Perfect Medical Health Management Limited is a multinational aesthetic medical corporate and one of the largest aesthetic medical companies in Hong Kong established in 2003. The Group focuses primarily on non-invasive aesthetic medical services and medical services in Hong Kong, China, Macau, Australia and Singapore with a total service area spanning approximately 322,000 square feet. Our operation offers a broad spectrum of professional services with assurance of utmost safety and efficacy. The Company was included as a constituent stock of the MSCI Hong Kong Small Cap Index on 27 May 2021, demonstrating the confidence of the capital market and recognizing the investment value of the Company.
Copyright 2022 ACN Newswire. All rights reserved. http://www.acnnewswire.com
SinoMab Received the Highest Subsidy from HKSTP

HONG KONG, Jul 8, 2022 – (ACN Newswire) – SinoMab BioScience Limited ("SinoMab" or the "Company", together with its subsidiaries, the "Group", stock code: 3681.HK), a Hong Kong-listed biopharmaceutical company dedicated to the research, development, manufacturing and commercialization of innovative therapeutics for the treatment of immunological diseases, primarily mAb-based biologics, is pleased to announce that, the Company has received HK$8 million subsidy from the Hong Kong Science and Technology Parks Corporation ("HKSTP"), which is the highest subsidy amount in the Clinical Translational Catalyst (CTC) program of HKSTP.
CTC program is offered by HKSTP Institute for Translational Research (ITR), the overarching aim is to provide a platform to transform Hong Kong and Greater Bay Area to become the go-to destination for translational medicine in the region. Under this program, funding support will be provided to biomedical companies of HKSTP to bring innovative, life-changing therapies and technologies to patients.
SinoMab stood out from many biopharmaceutical companies in the CTC program and was granted the highest subsidy amount, embodying the recognition by the evaluation committee on the Company's product candidates and research and development (R&D) plan. According to the agreement signed by both parties, HKSTP will provide SinoMab a subsidy of HK$8 million in the next 42 months in phases according to its clinical plan and progress for the clinical study of SN1011, the Company's key product, for the treatment of multiple sclerosis (MS).
SN1011 is SinoMab's key product and third-generation covalent reversible Bruton's tyrosine kinase (BTK) inhibitor. Following the approval of Investigational New Drug (IND) applications of SN1011 for systemic lupus erythematosus (SLE) and pemphigus vulgaris (PV) by the National Medical Products Administration (NMPA) on 27 August 2020 and 23 June 2021, respectively, the IND application of SN1011 for MS has been approved by the NMPA on 19 April 2022. The Company plans to initiate Phase II clinical study to evaluate the efficacy and safety of SN1011 in patients with MS in China and expects to enroll the first patient in the fourth quarter of 2022. The IND application of SN1011 for neuromyelitis optica spectrum disorder (NMOSD) was also accepted by the Center for Drug Evaluation (CDE) of the NMPA on 6 June 2022.
Dr. Shui On LEUNG, Chairman, Executive Director and Chief Executive Officer of SinoMab said that: "By launching the CTC program, HKSTP provides great support to local biopharmaceutical companies on their New Drug R&D. As a biopharmaceutical company raised in Hong Kong for 20 years, we are grateful for the long-term support and assistance of the HKSTP in promoting innovation and development of local biopharmaceutical companies. Previously, President Xi Jinping, and the Chief Executive of the HKSAR, Mr. Lee Ka-chiu, visited the Science Park, with the desire to forge Hong Kong into an international Innovation and Technology hub, demonstrating the country's high appreciation of Hong Kong innovative technology development. We are excited by the unprecedented opportunities. With the smooth progress of clinical trials of the Company's key candidates, the subsidy from the HKSTP will provide a solid foundation for the Company's continuous R&D and stepping toward commercialization. We will fully grasp the opportunity to accelerate the R&D and clinical trials of various products, further expand the product pipeline and potential indications, speed up the realization of product commercialization, adhere to the concept of independent innovation, strive for the well-being of patients and create value for shareholders."
About Hong Kong Science and Technology Parks Corporation
Established in 2001, HKSTP attracts and nurtures talent, accelerates and commercializes innovation and technology for entrepreneurs on their journey of growth in Hong Kong, to the Greater Bay Area, Asia and beyond. Its growing innovation ecosystem is built around its key locations of the Hong Kong Science Park in Shatin, InnoCentre in Kowloon Tong and three modern INNOPARKs in Tai Po, Tseung Kwan O and Yuen Long. The three INNOPARKs are realizing a vision of re-industrialisation for Hong Kong. The goal is sectors like advanced manufacturing, electronics and biotechnology are being reimagined for a new generation of the industry.
Through its infrastructure, services, expertise and network of partnerships, HKSTP will help establish innovation and technology as a pillar of growth for Hong Kong, while reinforcing Hong Kong's international I&T hub status as a launchpad for global growth at the heart of the GBA innovation powerhouse.
About SinoMab BioScience Limited
SinoMab BioScience Limited (stock code: 3681.HK) is dedicated to the research, development, manufacturing and commercialization of therapeutics for the treatment of immunological diseases. The Company's flagship product SM03 is a potential global first-in-target mAb against CD22 for the treatment of rheumatoid arthritis (RA) and is currently in Phase III clinical trial for RA in China, which has been recognized as one of the significant special projects of Significant New Drugs Development of the Twelfth Five-Year Plan Period and the Thirteenth Five-Year Plan Period. In addition, the Company possesses other potential first-in-target and first-in-class drug candidates, some of which are already in clinical stage, with their indications covering rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), pemphigus vulgaris (PV), non-Hodgkin's lymphoma (NHL), asthma, and other diseases with major unmet clinical needs.
Copyright 2022 ACN Newswire. All rights reserved. http://www.acnnewswire.com
Malaysian Genomics Proposes Private Placement

PETALING JAYA, Malaysia, Jul 8, 2022 – (ACN Newswire) – Malaysian Genomics Resource Centre Berhad (Bursa: MGRC, 0155), a leading genomics and biopharmaceutical specialist, announced today that the Group is proposing to undertake a private placement of up to 10% of its total number of issued shares or up to 12.42 million new placement shares at an issue price to be determined for third-party investors that will be identified later.
 |
Shareholders had approved in the AGM convened on 30 November 2021 for the Group to issue and allot new shares at any time and at such price as the Board of Directors deemed fit provided the number of new shares does not exceed 10% of the total number of issued shares. The new placement shares will rank equally in all respects with existing Malaysian Genomics shares except they will not be entitled to any dividends, rights, allotments and any other forms of distribution should the entitlement date precede the relevant date of allotment and issuance of the new placement shares.
Encik Azri Azerai, Executive Director of Malaysian Genomics, said, "We are proposing the private placement to raise funds mainly for the Group's future investments of which we are still exploring options and a part of the proceeds will be allocated for the purchase of equipment including IT hardware, biological safety cabinet and extraction automation for our existing and future businesses."
"The reopening of the economy presents opportunities in the private healthcare segment and the fresh funds raised will enable us to be able to take advantage of the possibilities. We diversified into the biopharmaceutical business in 2020 and have since been pushing to open channels for our cell therapies and genetic tests through a series of agreements that gives us a wider market reach. We also bought a 51% stake in a kidney dialysis operator, Aquahealth Sdn Bhd, to offer holistic kidney care."
UOB Kay Hian Securities (M) Sdn Bhd is the adviser and placement agent for the proposed private placement.
Malaysian Genomics Resource Centre Berhad: 0155 [BURSA: MGRC] [RIC: MGRC:KL] [BBG: MGRC:MK], http://www.mgrc.com.my/
Copyright 2022 ACN Newswire. All rights reserved. http://www.acnnewswire.com
Evolve Your Instinct with the Launch of Amazfit’s T-Rex 2

KUALA LUMPUR, Jul 7, 2022 – (ACN Newswire) – Amazfit, a premium smart wearable hardware brand owned by Zepp Health Corporation, launched the T-Rex 2, in Malaysia today, an outdoor, tough and rugged smartwatch, with a demonstration of its durability and versatility by wall climbing professionals from Camp5, the climbing gym.
 |
Amazfit recently sent the rugged outdoor GPS smartwatch Amazfit T-Rex 2 into space on a quest to prove its strength in extreme environments. The space journey began in the city of Sheffield in the United Kingdom, where Amazfit T-Rex 2 was sent off on a lightweight spacecraft equipped with fully functional tracking and control systems, as well as an on-board camera to capture the most exciting highlights during the two and a half hour round-trip.
The Amazfit T-Rex 2 has passed 15 military-grade tests and can remain functional in temperatures as low as -30 degrees Celsius through the ultra-low temperature operation feature. With a battery life that can stretch to 24 days, it comes with dual-band, 5-satellite positioning and route-import and real-time navigation for optimum outdoor use. Built for any terrain, the Amazfit T-Rex 2 can also resist heat up to 70 degrees Celsius, has a humidity resistance of 240h, 96h salt-spray resistance and shock-resistance.
Wearers can create their own training templates for 11 different sports through the Zepp app, which is powered by the Zepp operating system and includes a rich ecosystem of over 10 mini apps for seamless interactions. The Amazfit T-Rex 2 also has a huge selection of over 150 sports modes including the toughness-testing triathlon mode, the professional lap-data recording track run mode, with smart trajectory correction, and the more leisurely golf swing mode.
Mr. Benky Lin, Country Manager of Amazfit Malaysia, said, "There is no doubt that the Amazfit T-Rex 2 is one of the toughest smartwatches in the market. The military-grade tests ensures that it can withstand low and high pressure, extreme heat and cold, rain, salt, sand, dust, vibrations, shocks, acid and fungus. The Amazfit T-Rex 2 is built for durability and versatility and can be used for the great outdoors as well as for strength training in any condition."
"We are happy to introduce the Amazfit T-Rex 2 here in Malaysia. This is a smartwatch that is ready to evolve your instinct anywhere in the world. We have pushed it to the limit, going all the way to space, subjecting it to the most extreme conditions we can reach and that includes the heat of Death Valley in California and depths of the Pacific. The Amazfit T-Rex 2 is a smartwatch you can wear with confidence in any terrain condition."
The Amazfit T-Rex 2's look is inspired by the spirit of nature and comes in Astro Black & Gold, Ember Black, Wild Green and Desert Khaki. It is rugged in style with HD AMOLED display that enables the wearer to download dozens of watch faces with matching always-on displays or images unique to the wearer.
The Amazfit T-Rex 2 comes with one-year warranty and retails at RM799 each. To commemorate the launch, Amazfit T-Rex 2 will be exclusive to Shopee Online Store with a store promotional price from 6 July to 8 July. At the promo, there are also one each of Powerbuds Pro, GTS 2 mini and Band 5 to be won. The first 100 buyers of the T-Rex 2 will get an Amazfit sport bottle and screen protector as a gift.
To know more on the Amazfit T-Rex 2 series of smartwatches, please go to www.amazfit.com/en or to Amazfit's e-commerce partner platform http://bit.ly/Amazfit-MY-SHOPEE.
Amazfit: www.amazfit.com/en
Copyright 2022 ACN Newswire. All rights reserved. http://www.acnnewswire.com
Malaysian Genomics to Offer First-Ever Sports-Based Tests Specific to Southeast Asian Genetic Profiles

PETALING JAYA, Malaysia, Jul 6, 2022 – (ACN Newswire) – Malaysian Genomics Resource Centre Berhad (Bursa: MGRC, 0155), a leading genomics and biopharmaceutical specialist, is pleased to announce that the Group's wholly-owned subsidiary, MGRC Therapeutics Sdn Bhd (MGRCT), is collaborating with Rinani Genotec Sdn Bhd (Genotec) to offer sports-based genetic tests.
Genotec, a biomedical technology firm focusing on stem cell treatment and genetic testing, now offers a direct-to-consumer sports and fitness DNA test which enables individuals to better understand their genetic profile in sports and fitness-related areas. The test result includes categories such as energy metabolism and utilisation, muscle performance, response to cardiovascular training, weight management, nutrient metabolism, bone and joint health, and mental ability and performance. The information can then be integrated by individuals into their lifestyle in order to improve their daily sport and fitness performance.
The collaboration will see both parties consult and collaborate on sports-based genetic profile testing and the marketing of these services. In addition, Genotec will also work closely with MGRCT on the marketing of Malaysian Genomics' full range of Dtect genetic screening tests.
Dato' Alvin Joseph, Executive Director of MGRC, said, "This collaboration is another important step towards realising our vision of becoming a premier integrated life sciences company. By partnering with experts in sports science, we have now pioneered a genetic screening profile specifically designed for professional and amateur athletes, and everyday fitness enthusiasts. The science of genetics has a long history in professional sports and through this new profile we can support sportspeople in their pursuit of peak performance."
Mr. Julian Koay, Genotec's Director of Business Development, said, "With Malaysian Genomics' experience in genetics, what we have developed can be a game-changer for athletes and sports talents. We will use this new sports genetic profile to personalise fitness, sports and recovery regimes of sportspeople, to improve their performance."
Founders of Genotec, Julian Koay and Watson Chung, are the first certified DNA-based trainers in Malaysia, offering a unique programme combining knowledge of individuals' genetic profiles with a fitness or training regime that enhances the effectiveness of the fitness or training programmes.
Malaysian Genomics Resource Centre Berhad: 0155 [BURSA: MGRC] [RIC: MGRC:KL] [BBG: MGRC:MK], http://www.mgrc.com.my/
Copyright 2022 ACN Newswire. All rights reserved. http://www.acnnewswire.com
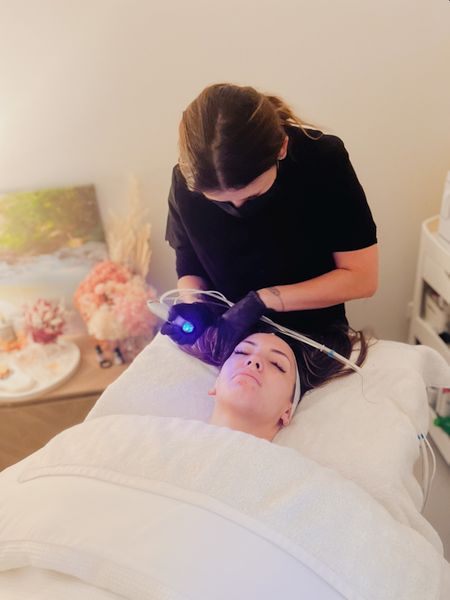
ReviewRumble 2022 Award Winners – Purapeel

SYDNEY, Jul 6, 2022 – (ACN Newswire) – PuraPeel Aesthetics wins the 2022 Award for best aesthetics business.
 |
The ReviewRumble annual awards recognize products and services that have been rated highly by the ReviewRumble community.
These awards are completely independent. Any listing can qualify to win an award as long as it meets the judging criteria below.
How are award winners determined?
To be eligible for an award, a product or a service must meet these requirements:
– Listed on the ReviewRumble website for consideration for an award
– In business for at least a year
– Minimum average rating of 4.1 stars online
– Ten or more approved reviews written and approved over the last five years
From this nomination list, the ReviewRumble team performs qualitative analyses and metric comparisons to calculate a 'sentiment score' to determine a category winner. In particular, the proportion of five-star to one-star reviews is taken into account, with more weight being given to reviews written in the last 12-months. In cases where the sentiment score is very close, we may award multiple listings within the category.
2022 Award Winner
PuraPeel Aesthetics is a clinic that offers skin treatment. Tania Quan is the founder of PuraPeel. Quan and her team discovered the PuraPeel treatment which is now one of the most popular treatments in Australia.
PuraPeel treatments provide deeper cleansing and extractions with suction that would otherwise have to be done manually.
About PuraPeel Aesthetics
A state-of-the-art facial treatment system that offers a multi-functional Hydra-Dermabrasion machine and advanced technology all in one.
PuraPeel Aesthetics components are made available to the highest specifications while partnering with industry leaders to create advanced medical-grade products that work in synergy with our PuraPeel devices.
Contact details:
Tania Quan
0449 788 289
https://www.purapeelaesthetics.com/
SOURCE: PuraPeel Aesthetics
Copyright 2022 ACN Newswire. All rights reserved. http://www.acnnewswire.com
