HONG KONG, Jul 4, 2022 – (ACN Newswire) – Perfect Medical Health Management Limited ("Perfect Medical" or the "Company", stock code: 1830.HK), one of the largest medical groups in Hong Kong, together with its subsidiaries (collectively referred to as the "Group"), is pleased to announce its annual results for the year ended 31 March 2022.
Performance highlights
— The Company achieved a historical high revenue of HK$1.35 billion, representing a growth of 23.9%.
— Among the value of sales contract, aesthetic medical accounted for 66.0%, medical business accounted for 17.4% and beauty and wellness accounted for 16.6%.
— The Company achieved a satisfactory net profit of HK$305.2 million despite the impact of the pandemic. If excluding the government subsidies, the revised net profit after tax increased by 29.8%.
— The Company has expanded its geographical coverage to Hong Kong, China, Australia and Singapore, representing total service area increased by 39.0% to 322,000 sq.ft..
— Basic earnings per share increased by 2.1% to HK24.8 cents.
— To reward the shareholders' unwavering support, the Board recommended the payment of a final dividend of HK7.1 cents per share. Together with the interim dividend of HK17.7 cents per share, the total dividend per share is expected to be HK24.8 cents per share for the full year, representing a total dividend payout ratio of 100.0%.
For the year under review, the Group's revenue increased by 23.9% to HK$1,350.0 million (FY2021: HK$1,089.8 million). The Group's EBITDA increased by 11.5% to HK$469.5 million (FY2021: HK$421.0 million). Profit attributable to shareholders of the Company was HK$305.2 million (FY2021: HK$284.6 million), representing a year-on-year increase of 7.2%, and a net profit margin of 22.6% (FY2021: 26.1%).
During the year, the Group has geared up its service centre expansion pace in Hong Kong, China and overseas. Currently, the Group has expanded its penetration in strategic locations at office premises and shopping malls, with total GFA increasing by 39% to 322,000 square feet.
Hong Kong Operation
Revenue from Hong Kong Operation increased by 43.6% to HK$975.1 million (FY2021: HK$679.0 million), mainly attributable to revenue contribution in the aesthetic medical and medical businesses as well as the additional revenue contributed from the new service centres established in the past years, but offset by the closure of all service centres for 84 days since January 2022 owing to the Omicron outbreak. Currently, the Group has a well established network of service centres in Hong Kong covering a total of 198,000 square feet.
The Company is mainly engaged in the operation of aesthetic medical and medical service in Hong Kong. From September to November 2021, the Company opened three service centres in Tsim Sha Tsui, Shatin and Central to consolidate its leading position in Hong Kong medical market.
In terms of the medical service business, leveraging on the strong foundation in the aesthetic medical business in Hong Kong, the Group has consistently reviewed its service portfolio through providing additional value-added services to enhance the customers' stickiness. In addition, the Group has made subsequent investments in a range of medical services to boost cross-selling and lower the acquisition cost of the customers.
Regions outside Hong Kong
Revenue from regions outside Hong Kong decreased by 8.8% to HK$374.8 million (FY2021: HK$410.8 million) due to the poor market sentiment and the continual lockdown under the pandemic. As of 31 March 2022, the Company has an extensive network in China, Macau, Sydney, Melbourne and Singapore, covering a gross service area of 124,000 square feet.
As one of the pioneers in the aesthetic medical industry in China, the Group has been focusing on key coastal first tier cities in Southern and Eastern China as well as the country's capital in Beijing, in order to cultivate a premium branding image. With the escalating customers' demand on a more all-round and professional medical services, the Group hopes to foster a stronger operation loop and a synergy to better serve our customers.
Prospects
Dr. Au-Yeung Kong, the executive director, chairman and chief executive officer of Perfect Medical, said that "This year marks the 11th anniversary of the Company's listing on the Stock Exchange of Hong Kong Limited since 10 February 2012. Our business covers Hong Kong, China, Macau, Australia and Singapore. At present, the Company has built a one-stop service platform incorporating a comprehensive aesthetic medical and medical services, fully catered to customers' needs.
With the weakening of the impact of the pandemic, the Company is well positioned to capitalize on the market opportunities and respond to the rebound of customers' demand in the post-pandemic era. In the future, the Company will gear up its effort organically, actively seek mergers and acquisitions of medical projects, and make optimisation and integration to offer additional high-quality services to our customers.
Looking ahead, the Company will increase the proportion of medical services and proceed with the international business expansion, with a view to becoming a truly multinational medical group.
For further information on the Group's FY2021/22 annual results, please refer to the Company's annual results announcement on the website of the Hong Kong Stock Exchange.
https://www1.hkexnews.hk/listedco/listconews/sehk/2022/0630/2022063000866.pdf
About Perfect Medical Health Management Limited
Perfect Medical Health Management Limited is a multinational aesthetic medical corporate and one of the largest aesthetic medical companies in Hong Kong established in 2003. The Group focuses primarily on non-invasive aesthetic medical services and medical services in Hong Kong, China, Macau, Australia and Singapore with a total service area spanning approximately 322,000 square feet. Our operation offers a broad spectrum of professional services with assurance of utmost safety and efficacy. The Company was included as a constituent stock of the MSCI Hong Kong Small Cap Index on 27 May 2021, demonstrating the confidence from the capital market and recognising the investment value of the Company.
Copyright 2022 ACN Newswire. All rights reserved. http://www.acnnewswire.com
Tag: Healthcare & Pharm
Tianda Pharmaceuticals Annual Results 2022

HONG KONG, Jun 30, 2022 – (ACN Newswire) – Tianda Pharmaceuticals Limited (Tianda Pharmaceuticals or the Group, stock code: 0455.HK) is pleased to announce its annual results for the year ended 31 March 2022. During the Reporting Period, the Group's business attained further development as revenue reached HK$510.0 million for the Current Financial Year, representing an increase of 7.5% compared to last year. Innovation and R&D made steady progress with R&D expenses increasing 34.7% year on year (YOY) to HK$15.9 million. With cash and cash equivalents of approximately HK$334.0 million as of 31 March 2022, the Group has sufficient financial resources to support its business development. To celebrate the 10th anniversary of the change of the Group's name to "Tianda Pharmaceuticals Limited", the Board of Directors recommended the payment of a final dividend of HK0.26 cent per share and a special dividend of HK0.56 cent per share to reward its shareholders for their support and trust over the years.
 |
 |
The Pharmaceuticals and medical technologies business revenue reached HK$396.1 million, an increase of 10.7% YOY. The Group focuses on products and technology, actively introducing, developing and acquiring innovative drugs, generic drugs, healthcare products, and medical devices for cardiovascular and cerebrovascular, women and children, and respiratory system diseases, oncology and rehabilitation. The Group's major product, Tuoping Valsartan capsules, a medicine for cardio-cerebrovascular disease, became the No. 1 product in its category in China by sales quantity as it seized the opportunity of the success in securing first place in the nation's Third Round of Centralized Drug Procurement with Target Quantity to supply nationwide, which increased both sales and brand awareness. Through the integration of sales teams and channels, the Group's medicines, especially the pediatric drugs, Tuoen Ibuprofen oral suspension and Ibuprofen suspension drops, also achieved satisfactory sales growth. The Group's new R&D and production base in Jinwan, Zhuhai has commenced operations, with an investment of HK$430 million for the first phase of the development and is equipped with imported and domestic advanced automated equipment. The new base is poised to become a pharmaceutical and health industry base with high standard, quality and efficiency through innovation development, accelerated product lines enrichment and production capacity improvement. Five CDMO/CMO contracts have been successfully signed.
The Chinese medicine business revenue reached HK$106.5 million, down 4.9% YOY. In the first nine months of the Reporting Period, the Chinese medicine business achieved faster growth by utilizing various methods to form mutually beneficial cooperation with partners such as medicinal materials farmers, cooperatives, distributors and pharmaceutical companies for building a nationwide and global business network for the Chinese medicinal materials business. Going forward, the Group will adopt a variety-centric approach to focus on domestic and overseas trading of Chinese medicinal materials, production and sales of TCM decoction pieces and formula granules, and distribution business, integrating quality resources from upstream to downstream for the industry.
The Medical and healthcare services revenue reached HK$7.4 million, an increase of 57.4% YOY. The Group steadily advances the development of the modern Chinese medical clinic "TDMall" through self-construction, franchising and mergers and acquisitions. The priority is to expand in the Guangdong-Hong Kong-Macao Greater Bay Area while making plans for a national and global rollout. TDMalls have been opened in Zhuhai, Hong Kong and Sydney successively since 2019 with the aim to build the global chain operation model under three different local laws and regulations for Chinese medicine. Meanwhile, Zhuhai TDMall became the first Chinese medicine clinic in the world to receive both the ISO 9001:2015 Quality Management System Certification and Qualicert International Service Quality Certification. As part of its caring for people's health and CSR initiatives, the Group launched the "TDMall Cloud-based Global Anti-epidemic Chinese Medicine Platform" amid the fifth wave of the COVID-19 pandemic in Hong Kong in early 2022 to support the Group's "Free Consultation and Medicine" charity campaign to provide the public with comprehensive remote Chinese medicine services from prevention, treatment to rehabilitation, as well as Chinese medicine services for mitigating long COVID.
China's "14th Five-Year Plan" proposes to comprehensively promote the construction of a healthy China, placing the protection of people's health as a strategic priority for development and providing people with comprehensive life-cycle health services. Adhering to the corporate slogan of "Tianda for Health!", the Group will continue to execute the strategy of "development of Chinese medicine business as a foundation, development of innovative drugs and medical technologies, as well as development of quality medical and healthcare services", implementing the "3D+1S" initiatives (business development (BD), research and development (R&D), investment and development (ID), and marketing & sales (S)) working in tandem to continuously enrich product lines and improve the quality and quantity of R&D projects in the pipeline through an external introduction, R&D, and mergers and acquisitions (M&A), as well as to identify cutting-edge technologies and products and quality projects worldwide in an effort to achieve high-quality development, so as to make a greater contribution to safeguarding the health of mankind.
About Tianda Pharmaceuticals Limited
Tianda Pharmaceuticals Limited is engaged in the development of the Chinese medicine business as a foundation, development of innovative drugs and medical technologies, as well as development of quality medical and healthcare services, committed to becoming a leading pharmaceutical enterprise that sets its footholds in China while expanding its presence worldwide.
For enquiries
Tianda Pharmaceuticals Limited
Investor Relations Department
Phone: +852 2545 3313
Email: ir@tianda.com
Copyright 2022 ACN Newswire. All rights reserved. http://www.acnnewswire.com
EC Healthcare and Yoho Group Form Strategic Partnership

HONG KONG, Jun 30, 2022 – (ACN Newswire) – EC Healthcare (the "Company", which together with its subsidiaries is referred to as the "Group", SEHK stock code: 2138), the largest non-hospital medical group in Hong Kong, announces that the Group signed a memorandum of understanding with Yoho Group Holdings Limited ("Yoho Group", SEHK stock code: 2347) for strategic collaboration. The partnership will be focused on accelerating the growth of medical, aesthetic medical and beauty and wellness services in the e-commerce market, and jointly boosting the e-commerce penetration of medical, aesthetic medical and beauty and wellness services in Hong Kong. The partnership will strengthen ECH's online presence in Hong Kong and expand its customer base and further consolidate the leading market position.
Under the collaboration, (i) The Group will become Yoho Group's anchor merchant in beauty and healthcare services and Yoho Group will launch an online medical, aesthetic medical and beauty and wellness service zone. The dedicated section will enable the Group to reach a greater public domain traffic flow from the e-commerce platform with strong client synergy in the healthcare and beauty sector;(ii)Both Parties will strengthen their co-marketing efforts to increase the overall sales volume and promote product cross-sell; (iii) Both Parties agree to exchange know-how in precision marketing and consumer behavior analysis in healthcare and beauty sector, in order to develop more comprehensive digitalized marketing strategy to improve overall customer lifetime value; (iv) Both Parties will devote resources to jointly enhance technical solutions across social & mobile commerce, data-driven marketing, and fulfillment efficiency to streamline the entire consumer journey.
Mr. Gemini Wong, Executive Director, Chief Digital Officer and Deputy Marketing Officer of EC Healthcare said, "The Group is delighted to establish a strategic partnership with Yoho Group. Through the collaboration, we hope to leverage Yoho Group's experience regarding online retail and the extensive reach to the accumulated customer traffic to boost the Group's e-commerce, diversify the digital marketing strategies and optimize the e-commerce experience so as to increase the overall sales volume, and raise the brand awareness and consolidate the leading position."
About EC Healthcare
EC Healthcare is Hong Kong's largest non-hospital medical service provider*, focusing on preventive and precision medicine and leveraging investment in IT, brand, service and corporate culture to build a diversified enclosed healthcare ecosystem with the mission to bring health, beauty and happiness to everyone. The Group is a constituent stock of the Hang Seng Composite Index and the MSCI Hong Kong Small Cap Index.
The Group principally engages in the provision of one-stop medical and health care services in Greater China. The Group provides a full range of services and products under its well-known brands, including those of its one-stop aesthetic medical solutions provider DR REBORN which has ranked first in Hong Kong by sales for years, a professional hair care center HAIR FOREST, primary care clinics jointly established with health management centre re:HEALTH, a vaccine centre Hong Kong Professional Vaccine HKPV, General outpatient clinic Tencent Doctorwork, the largest one-stop pain management centre in Hong Kong New York Medical Group, the comprehensive dental centres Bayley & Jackson Dental Surgeons, EC DENTAL CARE and Health and Care Dental Clinic, a advanced diagnostic and imaging centre HKAI, an oncology treatment centre reVIVE, a day procedure centre HKMED, a specialty clinic PREMIER MEDICAL CENTRE, SPECIALISTS CENTRAL and NEW MEDICAL CENTER, a paediatric centre PRIME CARE, a gynaecology specialist ZENITH MEDICAL CENTER AND PRENATAL DIAGNOSIS CENTRE, PathLab Medical Laboratories, Ophthalmology Center VIVID EYE and EC Veterinary Hospital and Imaging Center.
*According to independent research conducted by Frost and Sullivan in terms of revenue in 2020 and 2021
About Yoho Group Holdings Limited
Yoho Group Holdings Limited is one of the leading e-commerce platforms in Hong Kong, with more than 820,000 registered users as of the date of this announcement and over 2,290,000 monthly active users (based on the data in March 2022). According to Frost & Sullivan's data and the Group's revenue for the financial year ended 31 March 2021, the Group recorded the highest online retail sales among e-commerce players in consumer electronics and home appliances and acquired a market share of approximately 5.6%. Leveraging its vast customer base, the Group is expected to launch its online marketplace operations in the financial year ending 31 March 2023 to expand the product and service offerings for its customers.
Copyright 2022 ACN Newswire. All rights reserved. http://www.acnnewswire.com
Avantor Named Best Company for Upstream Processing and Single-Use Solutions at BMK 2022
Awards recognize Avantor innovations that allow the biopharmaceutical industry to bring therapies to market more efficiently
Incheon, Korea, June 30, 2022 – (ACN Newswire) – Avantor, Inc., a leading global provider of mission-critical products and services to customers in the life sciences, advanced technologies and applied materials industries, acquired awards for the best company in upstream processing and single-use solutions at the Biologics Manufacturing Korea (BMK) 2022 conference.

Sang Kyu Lee, Representative Director – Avantor Korea/Japan (Left), James Hwang, Head of Application, Asia, Middle East & Africa – Biopharma after receiving the Awards for Best Company in Single-use Solutions and Upstream Processing at BMK 2022
This is the second consecutive year that Avantor has been recognized for its upstream processing capabilities, a testament to its ongoing investment and innovations including development of high-purity raw materials, reagents and differentiated cell culture processes. In single-use solutions, Avantor received the best company award for the third consecutive year. Avantor was recognized for strengthening production capabilities and establishing an optimized supply chain in collaboration with global partners to respond to the growing need of biopharmaceutical manufacturers for single-use solutions.
Narayana Rao Rapolu, Vice President, Biopharma Asia Middle East & Africa for Avantor said, “Avantor’s single-use solutions enable biomanufacturers to bring new therapies to market faster and improve global health. Our local teams have expertise and experience across multiple processes that drive therapies forward from prototype development to system design, production and delivery. We offer the choice, agility and speed needed to support the evolving life-sciences market in Korea and across the region.”
Sang Kyu Lee, Representative Director for Avantor Korea & Japan said, “We are proud to be recognized for Avantor’s ability to support biopharmaceutical manufacturers through our comprehensive product portfolio and customer-driven production technologies. We continue to introduce innovations that allow life-changing therapies to reach the patients who need them.”
About Avantor
Avantor®, a Fortune 500 company, is a leading global provider of mission-critical products and services to customers in the biopharma, healthcare, education & government, and advanced technologies & applied materials industries. Our portfolio is used in virtually every stage of the most important research, development and production activities in the industries we serve. Our global footprint enables us to serve more than 225,000 customer locations and gives us extensive access to research laboratories and scientists in more than 180 countries. We set science in motion to create a better world. For more information, visit avantorsciences.com and find us on LinkedIn, Twitter and Facebook.
About The Korea Bioprocessing Excellence Awards
The Korea Bioprocessing Excellence Awards aims to recognize organizations within Korea that have engaged in substantial efforts to innovate, optimize processes and uphold a high level of efficacy, quality and safety in biological products manufacturing.
Regional Media Contact:
Christina Koh
Director, Communications – AMEA
Avantor
M: +65 9720 0169
Christina.Koh@avantorsciences.com
Global Media Contact:
Petro Kacur
Director, PR and External Communications
Avantor
M: 404-408-0663
Petro.Kacur@avantorsciences.com
Copyright 2022 ACN Newswire. All rights reserved. http://www.acnnewswire.com
Jacobson Pharma Announces FY2022 Annual Results

HONG KONG, Jun 29, 2022 – (ACN Newswire) – Jacobson Pharma Corporation Limited ("Jacobson Pharma" or the "Company"; Stock Code: 2633), a leading company engaged in the research, development, production, marketing and sale of essential medicines, specialty drugs and branded healthcare products, today announced the annual results of the Company and its subsidiaries (collectively the "Group") for the year ended 31 March 2022 ("FY2022" or the "Reporting Period").
KEY HIGHLIGHTS
— Year-on-year revenue up by 10.3%, amounting to HK$1,595.5 million
— Profit for the year up by 78.4%*, amounting to HK$187.7 million
— Strong growth witnessed on key therapeutic classes for chronic diseases such as lipid lowering agents and angiotensin II inhibitors
— Progress with expansion plan in Greater China as facilitated by Greater Bay Area ("GBA") healthcare policy and formation of joint venture with an established partner
— Delivery of approximately 9 million doses of Fosun BioNTech Comirnaty Vaccine in Hong Kong and Macau for the collaborative fight against COVID-19
During the Reporting Period, the Group delivered total revenue of HK$1,595.5 million with a year-on-year growth of 10.3%, primarily driven by the solid growth momentum of business in the public sector. The profit from operations and profit for the year was registered at HK$260.9 million and HK$187.7 million, representing a promising growth of about 48.9%* and 78.4%* respectively, as compared to the adjusted profit from operations* and adjusted profit* for the corresponding year of 2021.
The Group's financial position remains stable as supported by a healthy cash flow, with adjusted earnings before interest, taxes, depreciation and amortisation (adjusted EBITDA) posted at HK$441.6 million for the Reporting Period and a net gearing ratio at 29.2% as of 31 March 2022. With a cash balance of HK$478.7 million as of the end of the Reporting Period, the Group also recently secured a syndicated loan of HK$1.4 billion to enhance its capital capability.
The Board has recommended the payment of a final dividend of HK2.68 cents per share (FY2021: HK1.50 cents per share). Excluding the FY2021 special interim dividend in the form of distribution in specie of shares of JBM (Healthcare) Limited (Stock code: 2161.HK), an indirectly non-wholly owned subsidiary of the Company, total dividend for the Reporting Period amounts to HK3.88 cents per share (FY2021: HK2.30 cents per share), an increase of 68.7%.
Solid Performance of Generics Business
The Group's generic drugs business demonstrated a resilient performance amidst the COVID-19 pandemic, presenting a solid growth of 13.6%, with revenue posted at HK$1,191.3 million for the Reporting Period.
The Group's product offerings in the key therapeutic classes for chronic diseases such as diabetes and cardiovascular disease achieved high double-digit growth. For instance, the lipid-lowering product class recorded a strong growth of 37.2% in sales during the Reporting Period. In addition, the oncology drug class has also shown a significant growth of 572.6%, owing to the increased acceptance of the new Arsenic Trioxide Oral Solution.
As a major supplier of essential medicines in Hong Kong, the Group responded swiftly to cater to the surged demand for medications during the fifth wave of the epidemic. This was reflected by the strong growth in the Group's analgesics (+18.6%), cough & cold preparations (+45.8%) and anti-inflammatory products (+77.5%).
Steady Pipeline and Portfolio Enhancement
The Group continued to make steady progress with its research and development ("R&D") pipeline. As of 31 March 2022, the Group has a total of 172 products in the pipeline, among which 54 items have been approved for registration, 15 of them have been submitted for registration, 52 items have finished the development stage and are under stability preparation or stability study, plus 25 items are currently under formulation or pre-formulation research development stage.
As a continuous drive for portfolio enhancement, the Group launched a number of new products during the Reporting Period, including Rabeprazole Tablets, Valsartan and Amlodipine Tablets, Telmisartan and Hydrochlorothiazide Tablets, Pregabalin Capsules, Atosiban Injection, and Idarubicin Injection. Additionally, the Group has secured the registration approvals for a group of new products such as Levetiracetam Tablets, Febuxostat Tablet, Dexmedetomidine Infusion, Pramipexole Extended Released Tablets, Brimonidine and Timolol Eye Drops, Telmisartan and Amlodipine Tablets for upcoming market launches.
Tapping Specialty Drugs Market in China and Asia
Facilitated by new measures under the "Work Plan for Regulatory Innovation and Development of Pharmaceutical and Medical Devices in the Guangdong-Hong Kong-Macau Greater Bay Area", the Group has established collaboration with the University of Hong Kong-Shenzhen Hospital for the introduction of its Arsenic Trioxide Oral Solution (indicated for the treatment of acute promyelocytic leukaemia) into designated hospitals in the Greater Bay Area. The collaboration will also be part of a multi-centre clinical trial covering Guangdong, Singapore and Hong Kong.
More recently, the Group, together with its non-wholly owned subsidiary, JBM (Healthcare) Limited (Stock code: 2161.HK), formed a joint venture with Ban Loong Holdings Limited (Stock code: 0030.HK), a company of which Yunnan Baiyao Group Co., Ltd (Stock code: 0538.SZ) is the controlling shareholder, to capture the growth opportunities of the specialty medicines in Greater China and the Asia-Pacific region. The joint venture will be primarily engaged in exploring business opportunities in various growth streams including specialty medicines (including orphan drugs), over-the-counter drugs, and branded healthcare products as well as medical devices in the Greater China region.
Distribution of Fosun BioNTech Comirnaty Vaccine in Hong Kong and Macau
The Group is the exclusive distributor of Fosun BioNTech Comirnaty Vaccine (the "Vaccine") in Hong Kong and Macau. Up to the end of the Reporting Period, the Group delivered approximately 9 million doses of the Vaccine to the Department of Health and community vaccination centres in Hong Kong and the Macau governments.
To protect the public against COVID-19 and help ensure herd immunity through vaccination, the Group is committed to supporting the governments and professional partners in the acceleration of vaccination in Hong Kong and Macau, especially among the elderly, and will continue to collaborate with the Shanghai Fosun Pharmaceutical (Group) Co., Ltd. and its subsidiaries to supply booster vaccination doses for the public, as encouraged by the health authorities.
Mr. Derek Sum, Chairman and Chief Executive Officer of Jacobson Pharma, concluded, "While the pandemic continues to impact the market environment, the Group delivered a solid performance in FY2022 by focusing on strong execution, bolstered by our core capabilities and enhanced product pipeline and portfolio. We also take pride in playing a collaborative role in the distribution of the Fosun BioNTech Comirnaty Vaccine in Hong Kong and Macau and have demonstrated our commitment to robust manufacturing and logistics operations by ensuring a continuous supply of our essential medicines were delivered to hospitals and patients during Hong Kong's fifth wave of the epidemic.
"Looking ahead, we are entering 2022 with positive momentum as we aim to build a differentiated portfolio that anticipates future healthcare needs. By executing our R&D and in-licensing strategies, fostering strong partnerships, and cementing our foothold in key strategic markets, we will further diversify and transform our business, bringing sustainable growth and value to shareholders."
About Jacobson Pharma Corporation Limited (Stock Code: 2633)
Jacobson Pharma is a leading pharmaceutical company in Hong Kong vertically integrated and engaged in the research, development, production, sale and distribution of essential medicines and specialty drugs. As a major provider of generic drugs in Hong Kong, the Group has one of the most extensive sales and distribution coverage for both the private and public sectors in Hong Kong, with an expanding reach into strategically selected Asian markets. Carrying a broad product portfolio and taking a pre-eminent market position in a number of therapeutic categories, the Group operates a host of 10 PIC/S GMP licensed production facilities for generic drugs in Hong Kong.
The Group aims at the continued strategic enrichment of its generic drug portfolios through the addition of high-value-added products. With its corporate headquarters based in Hong Kong, the Group has also established its operating subsidiaries in China, Macau, Taiwan and Cambodia, forming a regional commercial platform to tap the market potential in the Asia Pacific and Greater China region. Jacobson Pharma has been a constituent stock of MSCI Hong Kong Micro Cap Index since 1 June 2017. For more details about Jacobson Pharma, please visit the Group's website: http://www.jacobsonpharma.com
* Excluding the one-off Employment Support Scheme subsidy from the Hong Kong Government of about HK$81.1 million in FY2021
Copyright 2022 ACN Newswire. All rights reserved. http://www.acnnewswire.com
Novotech Receives “Best Biologics CRO Award 2022” at Korea Bioprocessing Excellence Awards 2022

SEOUL, S.KOREA, Jun 29, 2022 – (ACN Newswire) – Novotech, the leading Asia Pacific biotech specialist CRO which has recently expanded its services to the US, was today awarded the Best Biologics CRO Award at a ceremony as part of 11th Biologics Manufacturing Korea, the 6th Cell & Gene Therapy World East Asia, and the 3rd BioLogistics World Korea conferences (29th – 30th June 2022) which attract more than 300 representatives from Korea's top biopharmas, vaccine manufacturers, and biologics companies.
Novotech Country Managing Director Sanghee Kim said the Korea team was extremely pleased to receive this award in recognition of the clinical excellence provided to clients in the biologics sector.
Novotech Vice President Global Head Clinical Services Yooni Kim also said: "Novotech's Asia-Pacific and US teams support cost-effective expedited clinical research with world-class data and the most advanced technology including solutions that enable acceleration of clinical trials across the regions."
Novotech now has a workforce of ~2,500 clinical trial professionals across Australia, New Zealand, South Korea, Greater China, Southeast Asia, India, South Africa and the US.
Novotech CEO Dr. John Moller said: "The focus on Asia-Pacific for biotech clinical research over the past five years makes the region the fastest-growing clinical trial destination with China being the leading location for new trials followed by the US. Asia-Pacific offers a compelling solution for expedited clinical trials especially in oncology with its vast patient populations, less competitive clinical trial landscape, and world-class KOLs, in addition regulatory reforms have accelerated approval processes. The expansion into the US provides US-based expertise and infrastructure for our US clients wanting trials in APAC and the US, and for our APAC clients wanting US clinical programs. Clients will receive a seamless service, with a unified approach to systems and SOPs," Moller said.
About Novotech Health Holdings
Novotech Health Holdings Pte. Ltd. ("Novotech") is the leading Asia-Pacific and US biotech specialist CRO. Novotech has integrated labs and phase I facilities and provides drug development consulting and clinical development services across all phases. It has been instrumental in the success of approximately 4,000 clinical trials across a broad range of therapeutic areas. Novotech is well-positioned to serve biopharma clients conducting clinical trials in Asia-Pacific and the US. For more information visit https://novotech-cro.com/contact
Media Contact
David James
E: communications@novotech-cro.com
AU: +61 2 8218 2144
USA: +1 415 951 3228
Asia: +65 3159 3427
Copyright 2022 ACN Newswire. All rights reserved. http://www.acnnewswire.com
Oculis Announces First Patient Enrolled in Phase 3 OPTIMIZE Trial of OCS-01 in the Treatment of Inflammation and Pain Following Cataract Surgery

LAUSANNE, Switzerland, Jun 29, 2022 – (ACN Newswire) – Oculis S.A., (Oculis) a global ophthalmology company developing life-changing treatments to save sight and improve eye care with breakthrough innovations, today announced that the first patient has been enrolled in its Phase 3 OPTIMIZE (Once-daily Post ocular surgery Treatment for InflaMmation and paIn to minimiZE drops) trial evaluating the efficacy and safety of once-daily OCS-01, a novel, high concentration, preservative-free, topical OPTIREACH formulation of dexamethasone for the treatment of inflammation and pain following cataract surgery.
In the completed Phase 2 SKYGGN study, once-daily OCS-01 successfully met its primary endpoint demonstrating superior efficacy and safety vs vehicle (placebo) in the treatment of inflammation and pain following cataract surgery. Positive data from that trial was presented at the American Society of Cataract and Refractive Surgery (ASCRS) 2020 Annual Meeting. Oculis subsequently held a positive end-of-Phase 2 meeting with U.S. FDA which enabled the start of the Phase 3 OPTIMIZE trial.
OPTIMIZE is a randomized, double-blind, placebo-controlled Phase 3 trial in 25 participating sites across the US and is scheduled to enroll approximately 240 patients. Efficacy measures of the trial include the absence of anterior chamber cells at Day 15 and absence of pain at Day 4.
Treatment of inflammation and pain following ocular surgery is another indication being pursued for OCS-01, following the commencement in November 2021 of the Phase 3 DIAMOND trial investigating OCS-01 in patients with DME.
Eric Donnenfeld, M.D. clinical professor of ophthalmology at New York University and Trustee of Dartmouth Medical School, said: "Following cataract surgery, patients often need to self-administer eye drops several times a day to manage inflammation and pain. An efficacious, preservative-free alternative, administered just once a day could provide significant advantages over current options."
Riad Sherif, M.D., CEO of Oculis, said: "This is another important development milestone for OCS-01, following the start of our Phase 3 trial in DME last year, which further signals the potential for this novel product candidate to address the limitations of currently available treatments for both retinal and front-of-the-eye indications. Clinical data generated so far have been very encouraging and we look forward to generating further data in this trial to support regulatory submissions."
OCS-01 has been developed using Oculis's OPTIREACH solubilizing nanoparticle technology, a proprietary platform that enables the formulation of drugs as non-invasive topical eyedrop treatments, a longer residence time on the eye surface and enhances their bioavailability in the relevant eye tissues, particularly the retina.
About Oculis
Oculis is a global biopharmaceutical company purposefully driven to save sight, improve eye care and address significant unmet medical needs with breakthrough innovations. Oculis's highly differentiated pipeline includes candidates for topical retinal treatments, topical biologics and disease modifying treatments. With a presence in key international markets, Oculis is poised to deliver life-changing treatments to patients worldwide. Headquartered in Lausanne, Switzerland and with operations in Europe, the U.S. and China, Oculis is led by an experienced management team with a successful track record and supported by leading international healthcare investors.
For more information, please visit: www.oculis.com
Copyright 2022 ACN Newswire. All rights reserved. http://www.acnnewswire.com
EC Healthcare Received “Institutional Investor” 2022 Multiple Awards in All-Asia Executive Team Rankings

HONG KONG, Jun 27, 2022 – (ACN Newswire) – EC Healthcare (the "Company", which together with its subsidiaries is referred to as the "Group", SEHK stock code: 2138), the largest non-hospital medical group in Hong Kong*, is pleased to announce the excellent result in the "All-Asia Executive Team Rankings 2022" under the category of Healthcare & Pharmaceutical organized by Institutional Investor, an international magazine.
 |
The Group is delighted recognize as The Most Honoured Company (Healthcare & Pharmaceutical) in the division – Asia (excluding mainland China), which represents recognition of the Group's impressive performance by the investors and the market.
Recognized as an authoritative ranking by industries, "All-Asia Executive Team Rankings" celebrates the outstanding companies and management teams in Asia. Buy-side and sell-side analysts rated the listed companies within the scope of their research according to several criteria used in evaluating performance in corporate governance.
Category / Award
Core Asia Results / Best IR Team
Asia ex-Mainland China:
The Most Honoured Company
Best CEO – Mr. Eddy Tang
Best CFO – Mr. Levin Lee
Best Investor Relations Program
Best ESG
Best Investor Relations Professional – Mr. Christopher Wong / Ms. Hermione He
Asia Small and Midcap:
Best CEO – Mr. Eddy Tang
Best CFO – Mr. Levin Lee
Best Investor Relations Program
Best ESG
Best Investor Relations Professional – Mr. Christopher Wong
Mr. Eddy Tang, Chairman, Executive Director and Chief Executive Officer of EC Healthcare said, "The Group is honoured and grateful to receive these awards. We will continue to spare tremendous effort with the aim of further consolidating the leading position in the healthcare market and maximizing our shareholder value. The Group wishes to excel together with our much-valued stakeholders and stay proactive in continuously refining our strategies for the overall welfare of our company and the greater community."
About EC Healthcare
EC Healthcare is Hong Kong's largest non-hospital medical service provider*, leveraging its core businesses of preventive and precision medicine, and committed to developing medical artificial intelligence by integrating its multi-disciplinary medical services. The move, which is supported by the Group's high-end branding and quality customer services, is aimed at offering customers safe and effective healthcare and medical services with professionalism. The Group is a constituent stock of the Hang Seng Composite Index and the MSCI Hong Kong Small Cap Index.
The Group principally engages in the provision of one-stop medical and health care services in Greater China. The Group provides a full range of services and products under its well-known brands, including those of its one-stop aesthetic medical solutions provider DR REBORN which has ranked first in Hong Kong by sales for years, a professional hair care center HAIR FOREST, primary care clinics jointly established with health management centre re:HEALTH, a vaccine centre Hong Kong Professional Vaccine HKPV, General outpatient clinic Tencent Doctorwork, the largest one-stop pain management centre in Hong Kong New York Medical Group, the comprehensive dental centres Bayley & Jackson Dental Surgeons, EC DENTAL CARE and Health and Care Dental Clinic, a advanced diagnostic and imaging centre HKAI, an oncology treatment centre reVIVE, a day procedure centre HKMED, a specialty clinic PREMIER MEDICAL CENTRE, SPECIALISTS CENTRAL and NEW MEDICAL CENTER, a paediatric centre PRIME CARE, a gynaecology specialist ZENITH MEDICAL CENTER AND PRENATAL DIAGNOSIS CENTRE, PathLab Medical Laboratories, Ophthalmology Center VIVID EYE and EC Veterinary Hospital and Imaging Center.
*According to independent research conducted by Frost and Sullivan in terms of revenue in 2020 and 2021
Copyright 2022 ACN Newswire. All rights reserved. http://www.acnnewswire.com
Novel patching material for bone defects

TSUKUBA, Japan, Jun 27, 2022 – (ACN Newswire) – Ceramics and metals have been used for a while as structural materials to repair bones and joints. In the past, scientists engineered bioinert materials, which do not bond to bones directly; bioactive materials that can bond to bones; and bio-absorbable materials that are categorized in bioactive materials but they are absorbed by the body over time and are replaced by advancing bone tissue.
 |
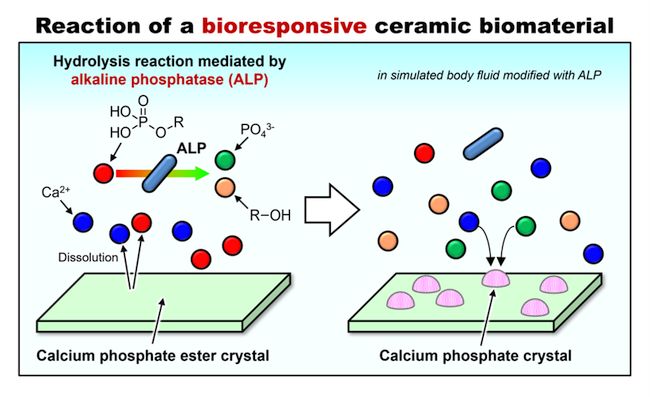 |
Now, a fourth type of bone repairing materials has been found: a bio-responsive ceramic that interacts with an enzyme found in blood to be absorbed into the body at a precise and predictable rate.
The research was done by Taishi Yokoi, an associate professor at the Institute of Biomaterials and Bioengineering at Tokyo Medical and Dental University, and his colleagues. The study was published in May in Science and Technology of Advanced Materials.
"Extending healthy life expectancy is an important issue for all of us," Yokoi says. "Bone repairing materials aid in the recovery of bone defects and help improve quality of life."
At the heart of this discovery is a biological reaction: an enzyme called alkaline phosphatase (ALP), which is present in human serum and reacts with various phosphate esters to generate bone mineral known as hydroxyapatite.
The scientists mimicked this process using a simulated body fluid that contained the enzyme ALP. They placed four different salts in a simulated body fluid containing or lacking the enzyme ALP. The salts were calcium salts of methyl phosphate (CaMeP), ethyl phosphate (CaEtP), butyl phosphate (CaBuP) and dodecyl phosphate (CaDoP). The phosphate component of each of these salts has an alkyl group at its end – a chain composed of hydrogen and carbon atoms – of differing lengths.
The scientists found that the first three salts were converted to hydroxyapatite, but only in the presence of ALP. Interestingly, the length of the alkyl group on the phosphate ester determined the rate at which this reaction happens. With more research, the scientists think that this could allow greater control of the bone healing process in the body.
"We expect the findings of this study will be applied towards designing and developing novel bone-repairing materials with precisely controlled degradation and resorption rates inside the body," says Yokoi.
Further information
Taishi Yokoi
Tokyo Medical and Dental University
Email: yokoi.taishi.bcr@tmd.ac.jp
Research paper: https://www.tandfonline.com/doi/full/10.1080/14686996.2022.2074801
About Science and Technology of Advanced Materials (STAM)
Open access journal STAM publishes outstanding research articles across all aspects of materials science, including functional and structural materials, theoretical analyses, and properties of materials. https://www.tandfonline.com/STAM
Mikiko Tanifuji
STAM Publishing Director
Email: TANIFUJI.Mikiko@nims.go.jp
Press release distributed by Asia Research News for Science and Technology of Advanced Materials.
Copyright 2022 ACN Newswire. All rights reserved. http://www.acnnewswire.com
Novotech Receives CRO Leadership Award for Exceeding Customer Expectations

CHICAGO, IL, Jun 23, 2022 – (ACN Newswire) – Novotech, the leading Asia Pacific biotech specialist CRO which has recently expanded its services to the US, was awarded a prestigious Clinical Leader and Life Science Leader CRO Leadership Award for exceeding customer expectations, at Drug Information Association 2022 (DIA 2022).
 |
Novotech CEO Dr. John Moller said the company was extremely pleased to receive this award for the second consecutive year. "Receiving this recognition from our clients is incredibly important to us and is an endorsement of our service delivery model that is tailored to the needs of biotech clients. Our local teams have exceptional site and investigator access, our project management approach emphasises problem solving, ownership and flexibility, and our investments in data and technology ensure clients have real time access to trial performance."
Book a meeting with one of the Novotech experts at DIA here. https://novotech-cro.com/contact
Clinical Leader and Life Science Leader working with Industry Standard Research (ISR), selected Novotech for the CRO Leadership Award.
According to the CRO Leadership Award research team: "The awards are based on customer feedback. Winning CROs are chosen through impartial market research based on feedback from sponsor companies that utilize outsourcing services. Primary market research by ISR Reports is the basis of the awards. Sponsors provide ratings of CROs based on recent outsourced projects. This experiential feedback is analyzed by sponsor company size to reveal leading CROs in different performance categories."
Ed Miseta, Chief Editor for Clinical Leader said: "Selecting the right CRO can make or break your project. It can lead to a successful regulatory submission and approval or cost you a lot of time and effort on a failed study. That makes CRO selection a stressful decision for any clinical operations manager. Regardless of whether you are concerned about compatibility, capabilities, expertise, quality, or reliability. We believe our CRO Leadership Awards will help managers with their search process and hopefully help to connect them with the right contract partner. We are grateful to our colleagues at ISR Reports for conducting the research necessary to produce these awards. These award winners have proven themselves to be the top service providers in each category. I congratulate all of them for the work ethic they exhibit in consistently meeting the needs of their drug development clients."
Kevin Olson, CEO of Industry Standard Research said: "Industry Standard Research (IRS) continues to consider it an honor to provide the primary market research data for Life Science Leader and Clinical Leader's CRO Leadership Awards. ISR's stringent screening process ensures that only highly qualified industry decision-makers participate in our CRO benchmarking market research. This is paramount as we ask the research participants to provide experiential, not perceptual, feedback on their involvement with contract suppliers over the past 18 months. The data enable users of ISR's market research to make confident business decisions based on the experiences of their industry peers."
Novotech, which has a reputation for delivering full-service, high-quality expedited clinical trials in Asia-Pacific, can now offer its biotech clients clinical services in the US to support later phase global studies. Novotech now has a workforce of ~2,500 clinical trial professionals across Australia, South Korea, Greater China, Southeast Asia, India, South Africa and the US.
Novotech CEO Dr. John Moller said Novotech's Asia-Pacific and US teams support cost effective expedited clinical research with world-class data, and the most advanced technology including solutions that enable acceleration of clinical trials across the regions. "The focus on Asia-Pacific for biotech clinical research over the past five years makes the region the fastest growing clinical trial destination with China being the leading location for new trials followed by the US. Asia-Pacific offers a compelling solution for expedited clinical trials especially in oncology with its vast patient populations, less competitive clinical trial landscape, and world-class KOLs, in addition regulatory reforms, such as those in China, have accelerated approval processes. The expansion into the US was a strategic move to provide US-based expertise and infrastructure for our US clients wanting trials in APAC and the US, and for our APAC clients wanting US clinical programs."
About Novotech Health Holdings Pte Ltd ("Novotech")
Novotech is the leading Asia-Pacific and US biotech specialist CRO. Novotech has integrated labs and phase I facilities and provides drug development consulting and clinical development services across all phases. It has been instrumental in the success of approximately 4,000 clinical trials across a broad range of therapeutic areas. Novotech is well-positioned to serve biopharma clients conducting clinical trials in Asia-Pacific and the US. For more information visit https://novotech-cro.com/contact
Media Contact
David James
E: communications@novotech-cro.com
AU: +61 2 8218 2144
USA: +1 415 951 3228
Asia: +65 3159 3427
Copyright 2022 ACN Newswire. All rights reserved. http://www.acnnewswire.com
