
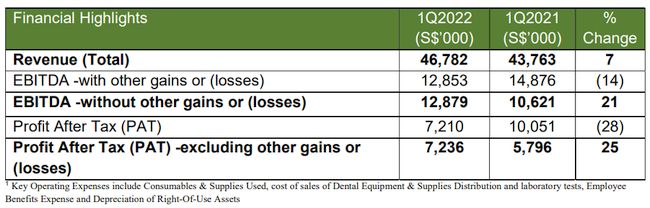
SINGAPORE, May 12, 2022 – (ACN Newswire) – Q&M Dental Group (Singapore) Limited, a leading private dental healthcare group in Asia, reported net profit excluding other gains or losses attributable to shareholders of S$7.2 million for the quarter ended 31 March 2022 (1Q2022) compared to S$5.8 million in the corresponding period (1Q2021).
 |
Dr Ng Chin Siau, Chief Executive Officer of Q&M Dental said, "We are heartened by our continuous strong performance in 1Q2022 following a record-setting year FY2021. Our core dental business will remain strong, and with the abolishing of most of the pandemic restrictions, a positive impact on the economy, we are looking forward to resuming our growth trajectory of expanding our network of clinics in the coming quarters."
Growth & Expansion in Operations
As at 31 March 2022, the Group's number of dental outlets in Singapore has grown to 99, from 85 as at 31 March 2021. Similarly in Malaysia, the number of dental outlets has increased to 41, as compared to 36 previously.
The Group also continues to focus on its expansion into medical diagnostics, with its associated Company, Aoxin Q&M Dental Group Limited and through its recent acquisition of 49% shareholding of Acumen Diagnostics Pte. Ltd., raising Q&M's effective interest in Acumen Diagnostics from 51% to 67%. In the near term, Acumen Diagnostics' clinical testing laboratory will roll out its pipeline of new tests, including PCR assays for dengue, sepsis and, identification of bacterial pathogens and their associated antibiotics resistance in pneumonia and bloodstream infections. Its longer-term plans include the development of new vaccines.
For 1Q2022, revenue contribution from dental and medical clinics was relatively unchanged with a marginal decrease of 2% to S$38.6 million, despite the challenges of the tightening of Safe Management Measures (SMM) due to the rapid spread of the Omicron variant. Revenue contribution from medical laboratory and dental equipment & supplies segment surged by 80% to S$8.2 million on the back of increased revenue from Covid-19 testing from Acumen Diagnostics, the Group's medical laboratory business, which obtained its medical laboratory license in September 2020.
The Group's net profit after tax excluding other gains or losses for 1Q2022 increased by 25% to S$7.2 million, from S$5.8 million in the corresponding period last year, translating to an earnings per share of 0.69 Singapore cent.
As at 31 March 2022, the Group had cash and cash equivalents of S$43.5 million with net assets of S$94.9 million. This translates to Net Asset Value per share of 10.10 cents per share.
Outlook & Further Expansion Plans
With the opening up of the Singapore economy gathering pace in 2022, the country expects to see businesses ramping up in most sectors with those that were most severely affected by the pandemic like F&B, tourism to especially benefit. The government is forecasting GDP growth of between 3.0 to 5.0% in 2022, Barring any major changes in the global macroeconomic and political situation or any new adverse developments in the evolving Covid-19 situation, the Group is cautiously optimistic on its business outlook.
The Group intends to continue executing the business plans outlined below:
– Expansion of network of dental clinics in Singapore and Malaysia
The Group currently operates 99 clinics in Singapore. With the economy opening up in 2022, the Group will also intensively increase its reach through organic growth of its dental clinic network in Singapore. This will be supported by an expansion of its team of dentists to undergird the future growth of its operations in Singapore. We will continue to develop, invest and optimise our digital Artificial Intelligence (AI) guided clinical decision support system to provide the most effective and suitable treatment plans for patients. The Group believes it is well-positioned to cater to the rising demand for primary and high-value specialist dental healthcare services of its patients.
Currently, the Group operates 41 clinics in Malaysia. The clinics are located in Johor (16 dental clinics), Kuala Lumpur (9 dental clinics), Selangor (11 dental clinics), Melaka (4 dental clinics) and 1 dental clinic in Negeri Sembilan.
The Group intends to open at least 30 dental clinics a year from 2021 onwards in Singapore and Malaysia for the next 10 years. The eventual number of dental outlets will depend on available opportunities, pertinent market conditions and the evolving Covid-19 situation.
– Expansion into private dental healthcare market in the People's Republic of China ("PRC")
The main thrust of the Group's proposed expansion in PRC is through organic growth to develop a new and sustainable growth pillar that can yield long term value for the Group.
– Expansion in Southeast Asia
The Group is continuously looking out for strategic opportunities to expand its dental business and regional footprint to other Southeast Asian countries.
– Medical Laboratory
The Group will focus on rolling out its clinical testing laboratory pipeline of new tests including PCR assays for dengue sepsis and identification of bacterial pathogens and their associated antibiotics resistance in pneumonia and bloodstream infections.
Please see links for PDF documents from SGXNET.
Results: https://tinyurl.com/QnM-1Q2022-Result
Press Release: https://tinyurl.com/QnM-1Q-2022-Release
About Q&M Dental Group (Singapore) Limited (QC7.SI)
Q&M Dental Group (Singapore) Limited (QC7.SI) ("Q&M" or together with its subsidiaries, the "Group") is a leading private dental healthcare group in Asia.
The Group owns the largest network of private dental outlets in Singapore, operating 99 dental outlets across the country. Underpinned by about 290 experienced dentists and over 350 supporting staff, the Group sees an average of 40,000 patient visits a month in Singapore. The Group also operates 5 medical clinics and a dental supplies and equipment distribution company.
Outside of Singapore, the Group has 41 dental clinics and a dental supplies and equipment distribution company in Malaysia, as well as a dental clinic in the People's Republic of China ("PRC"). Q&M is also the substantial shareholder of Aoxin Q&M Dental Group Limited, a dental Group listed on the Catalist board of the Singapore Exchange that operates dental clinics and hospitals primarily in the north- eastern region of the PRC. The Group aims to expand its operations geographically and vertically through the value chain in Malaysia, the PRC and within the ASEAN region.
The Q&M College of Dentistry was established in 2019 to offer postgraduate dental education as part of its commitment to continual education and professional development of dentists. It offers Singapore's first private postgraduate diploma programme in clinical dentistry.
In 2020, the Group expanded into the medical laboratories and research industry with the strategic investment into Acumen Diagnostics Pte. Ltd. ("Acumen"). Acumen currently focuses on the manufacture, sale and distribution of COVID-19 diagnostic test kits, as well as COVID-19 testing. It is also working to roll out a pipeline of new tests, including PCR assays for dengue, sepsis and, identification of bacterial pathogens and their associated antibiotics resistance in pneumonia and bloodstream infections.
EM2AI Pte Ltd, a wholly-owned subsidiary of the Group that focuses on developing AI-powered solutions to diagnosis and treatment planning has rolled out IDMS, enabling dentists within the Group's network to administer ethical treatment plans for patients.
The Group was listed on the Mainboard of the Singapore Exchange Securities Trading Limited ("SGX- ST") on 26 November 2009. For more information on the Group, please visit www.QandMDental.com.sg
For more information, please contact:
Waterbrooks Consultants Pte Ltd
Wayne Koo
Tel: +65 9338-8166
Email: wayne.koo@waterbrooks.com.sg
Derek Yeo
Tel: +65 9791-4707
Email: derek@waterbrooks.com.sg
Copyright 2022 ACN Newswire. All rights reserved. http://www.acnnewswire.com












